The Science of ECA
The science behind ECA, known as electrochemistry, has been generally known since 1834, with Michael Faraday publication The Laws of Electrolysis. On a very basic level, electrochemistry deals with the interactions between electrical energy and chemical change. In electrolysis, an electrical current drives chemical reactions known as oxidation and reduction--the very properties that make anolyte and catholyte such powerful cleansing and sanitizing agents--that otherwise would not occur spontaneously.
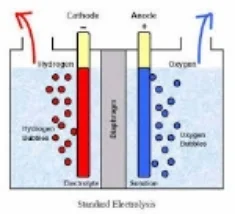
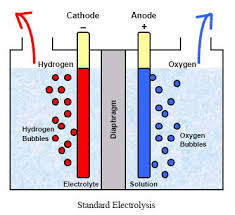
The key to ECA can be thought of in terms of the transfer of electrons. The process is similar to that of charging a battery. The difference is that batteries store their energy in an acid, whereas with water electrolysis, all of the energy is consumed by the water and salt molecules forcing electro-chemical changes that result is the generation of natures most perfect cleaners and sanitizers. Perfect - because they are both effective and non-toxic. When all of the water evaporates, all that remains is a tiny salt crystal. When water from chemical soaps or sanitizers evaporates, they leave behind toxic film like residues all of which are ideal habitats for one or more biofilms to colonize. The actual ECA process involves submerging two differently charged electrodes, on positive (anode) and one negative (the cathode) into an ionized solution which is, in this case, salt water. An ionized solution is simply any solution with positively and negatively charged ions, termed anions and cations. The positive anode attracts the negative anions of chloride and hydroxide and the negative cathode does the same for the positive sodium and hydrogen cations. This “attraction” of opposites involves the transfer of negatively charged electrons triggering the important oxidation-reduction reaction, wherein the net gain of electrons is known as reduction and the net loss is oxidation.
Specifically, the chloride ions give up two electrons to the anode and combine to make chlorine gas, which is contaminated by small amounts of oxygen due to the same oxidizing behavior of hydroxide (there are far more chloride ions, which is why they dominate the oxidation reaction).
Meanwhile, on the cathode side, the hydrogen ion gains electrons to become hydrogen gas and there is an accumulation of sodium ions, which combine with the newly formed hydroxide to form a sodium hydroxide solution.
2 Cl-(aq) - 2e- ➤ Cl2(g)
2 Cl-(aq) - 2e- ➤ Cl2(g)
As hydrogen gas forms, however, the water equilibrium shifts to the right, creating more hydroxide ions.
2H+(aq) + 2e- ➤ H2(g)
A semi-porous "dielectric" membrane separates the anode side of the cell from the cathode side, regulating the potential difference between the hydrogen and hydroxide ions, and also allowing for the venting of hydrogen gas at the cathode side and oxygen gas by the anode. Tiny micro, nano and pico bubble of gas are formed at the electrode interface, and cold plasma affects reign heavily along gas/liquid interface. The membrane prevents the build up of gaseous chlorine (the chlorine gas manufactured at the anode, becomes elemental chlorine with the release of oxygen gas). In addition, the membrane allows for the combination of this chlorine with hydroxide ions, creating a hypochlorous acid solution in the anolyte chamber.
THE MECHANISM OF ACTION
Catholyte is an excellent surfactant: sodium hydroxide’s opposing hydrophobic and hydrophilic molecular chains act to essentially tear the bonds between soils apart. Moreover, because the hydrophilic, polar head causes a decrease in the surface tension of water, catholyte is able to penetrate deep into embedded soils, such as mold. By adding various forms of kinetics, the effectiveness of the process can be improved exponentially.
The oxidizing potential and ionic activity of anolyte kills bacteria by rupturing the cell wall and then denaturing its extracellular polymerace substances (EPS). In a similar fashion, there is an abundance of free radicals in freshly generated anolyte. These highly reactive free radicals are known to rapidly denature DNA, RNA and proteins. Our ECA anolyte has been shown by NOROcore (https://norocore.ncsu.edu/) to inactivate live human norovirus. At the time of this writing (April 2016), our ECA anolyte is the only substance tested that is known to do so.